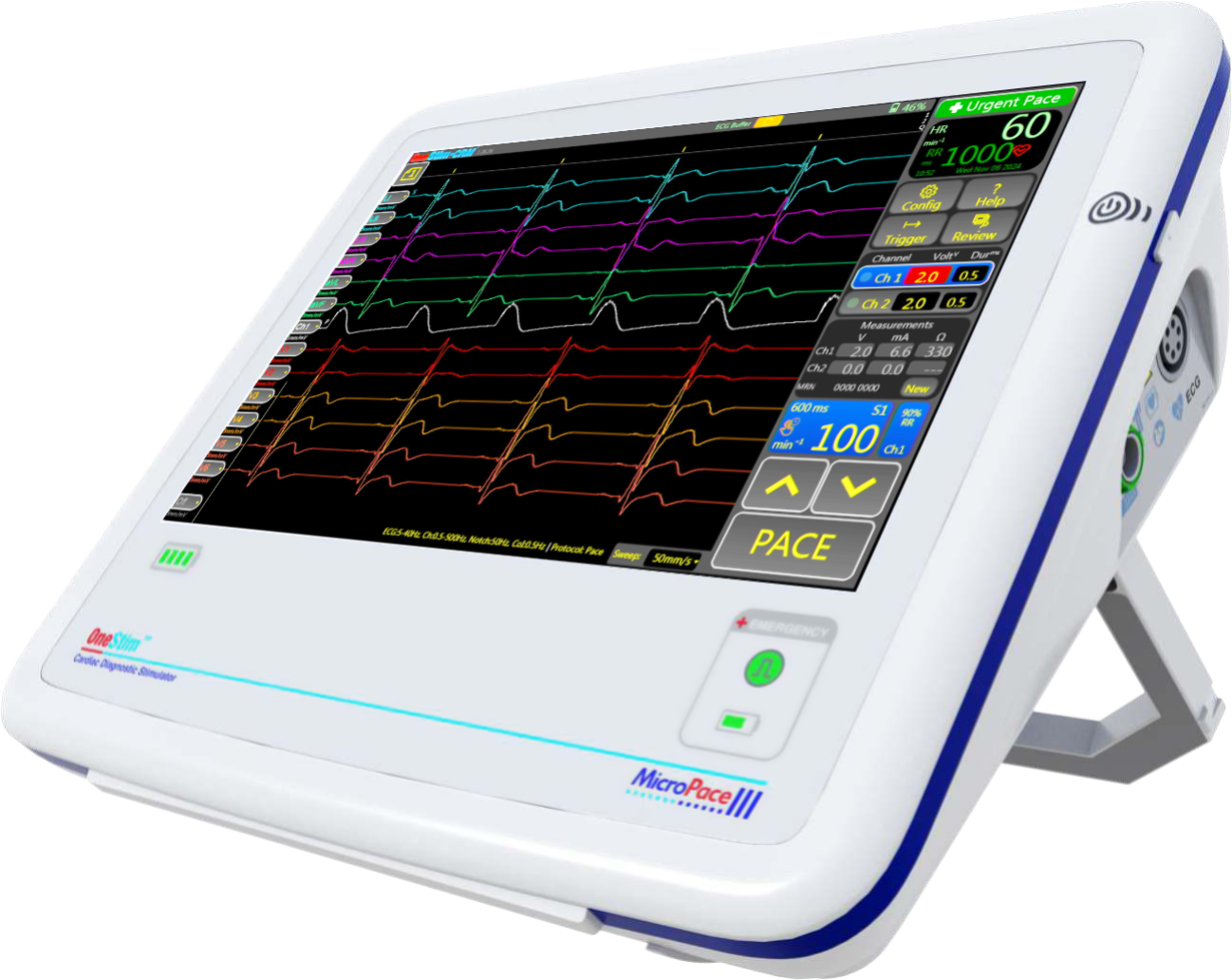
Pace, Measure, Analyse
OneStim™ CRM
The OneStim-CRM™ provides, in one device, the key functionality recommended for implanting conduction system pacing leads according to the 2023 EHRA clinical consensus statement on conduction system pacing implantation [1], namely:
- Voltage controlled pacing
- Continuous impedance measurements
- Current of Injury display, and
- 12 Lead ECG display and measurement.
Features
Continuous impedance with graph
12 ECG display
Trigger screen with semi-automatic measurements - RWPT, V6-V1 Int
Paced extras
Template comparison
Review screen with multiple callipers
OneStim™ CRM Specifications
Patient Interface
- 2 intra-cardiac channels
- 12-13 lead surface ECG
- 1 independent emergency pacing channel
Programmable Stimulation
- Pace, A-V Delay, Nodal ERP R-synced Sx, Threshold
- 0.1-25V (50V in esophageal mode)
- 0.1-25mA
ECG Analysis & Reporting
- Up to 90s buffer of saved ECG for review at sweep speed up to 400mm/s
- Time and amplitude measurement callipers (±1ms, ±0.1mV)
- Triggered sweep
- Template comparison
- Save printable reports
Training Videos
Reference:
[1] Burri, H., Jastrzebski, M., Cano, Ó., Čurila, K., de Pooter, J., Huang, W., Israel, C., Joza, J.,
Romero, J., Vernooy, K., Vijayaraman, P., Whinnett, Z., & Zanon, F. (2023). EHRA clinical consensus
statement on conduction system pacing implantation: endorsed by the Asia Pacific Heart Rhythm Society
(APHRS), Canadian Heart Rhythm Society (CHRS), and Latin American Heart Rhythm Society (LAHRS). Europace,
25(4), 1208–1236.
